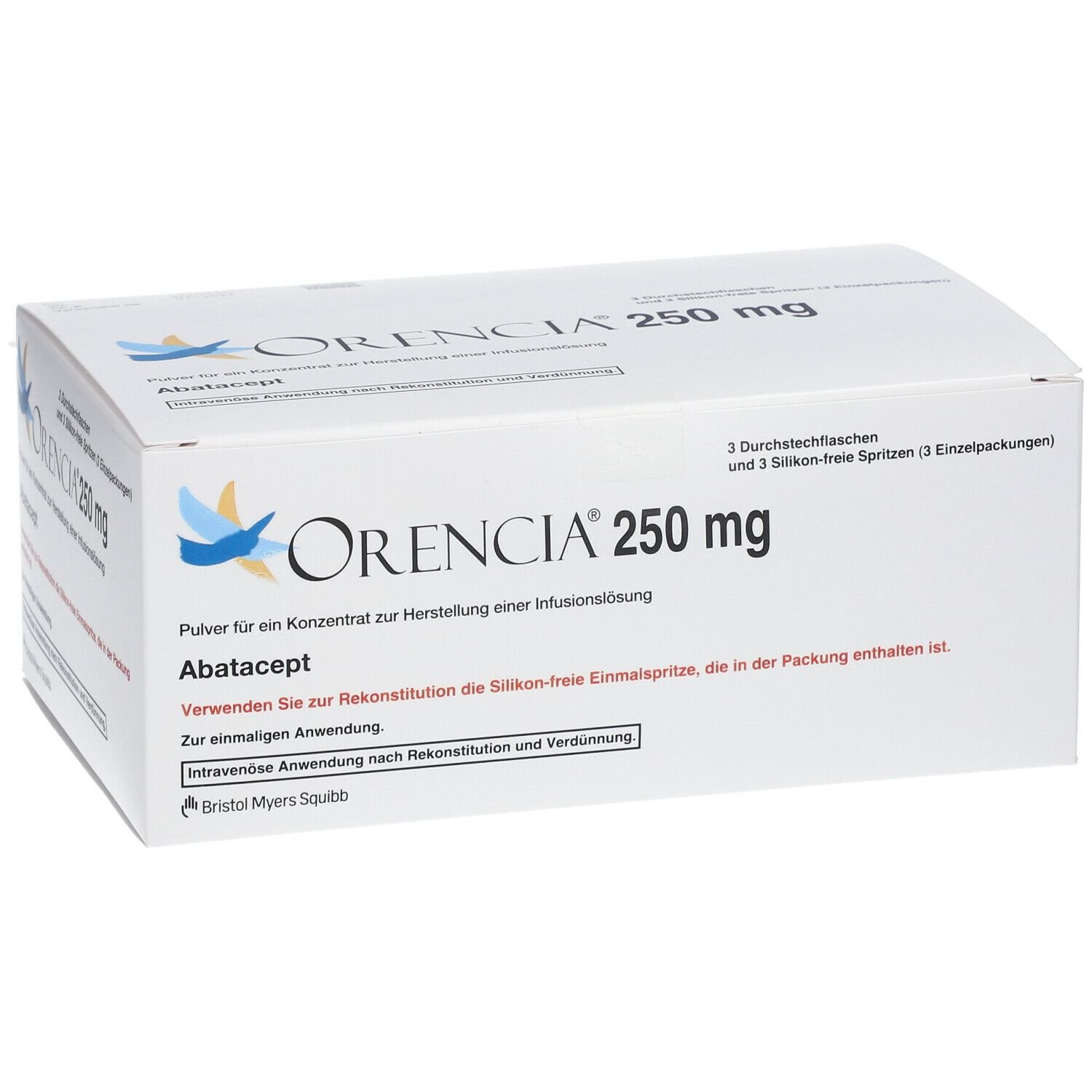
Description
ORENCIA 250mg (abatacept) is a medication used primarily in the treatment of rheumatoid arthritis (RA) and polyarticular juvenile idiopathic arthritis (JIA). Here are the key indications and considerations for its use:
Indications
- Rheumatoid Arthritis (RA) in Adults:
- Moderate to Severe Active RA: Used in combination with methotrexate (MTX) for adult patients who have not responded adequately to previous therapy with one or more disease-modifying anti-rheumatic drugs (DMARDs) including MTX or a TNF-alpha inhibitor.
- Highly Active and Progressive Disease: Also indicated for adult patients with RA who have not previously been treated with methotrexate.
- Polyarticular Juvenile Idiopathic Arthritis (JIA) in Pediatric Patients (6 years and older):
- Used in combination with other DMARDs, including at least one TNF inhibitor, for the treatment of moderate to severe active polyarticular JIA in pediatric patients who have had an inadequate response to previous DMARD therapy.
Mechanism of Action
- Abatacept works by inhibiting T-cell activation, which plays a crucial role in the immune-mediated inflammatory processes involved in RA and JIA.
Safety Considerations
- Driving and Machine Use: While abatacept is expected to have minimal impact on the ability to drive or operate machinery, dizziness has been reported as a common side effect. Reduced visual acuity has been reported uncommonly. Patients experiencing these symptoms should avoid driving or using machinery until the symptoms resolve.
Adverse Reactions
- Common Side Effects: Dizziness
- Uncommon Side Effects: Reduced visual acuity
Administration
- ORENCIA 250mg is typically administered intravenously (IV) and is used in combination with methotrexate or other DMARDs as directed by healthcare providers experienced in the treatment of RA and JIA.
Conclusion
ORENCIA 250mg (abatacept) is an effective treatment option for moderate to severe active RA in adults and polyarticular JIA in pediatric patients. It is used in combination with methotrexate or other DMARDs when previous therapies have not provided adequate relief. Patients should be monitored for common and uncommon adverse effects, and precautions should be taken if dizziness or reduced visual acuity occurs. Treatment decisions should always be made in consultation with a healthcare provider knowledgeable about autoimmune conditions and their treatments.



Reviews
There are no reviews yet.